What is ALS?
Amyotrophic lateral sclerosis (ALS) is the most common type of adult-onset motor neuron disease.1 It is a severe neurodegenerative disorder that results from progressive loss of neurons in the central nervous system.1–3
The death of motor neurons, which control voluntary movement in the motor cortex of the brain and in the spinal cord, causes progressive muscle weakness and functional decline, including loss of the ability to walk, speak, and swallow.1,2 As the disease progresses, a total loss of motor function eventually leads to paralysis and subsequent death.1,4
Currently, there is no cure for ALS, although 2 medications – riluzole and edaravone – have been shown to have some efficacy in slowing disease progression.5,6 As such, most therapeutic strategies are holistic and primarily aimed at alleviating symptoms.6
What causes ALS?
The pathogenesis of ALS is complex and is not completely understood, and both environmental and genetic factors have been implicated in the development of the disease.6,7 Risk is higher in individuals of male sex and of increased age.8 Some genetic variations may indicate an increased risk of developing ALS.6 Pre-disposing genetic factors include ATXN2 intermediate repeat expansions and mutations in UNC13A, an at-risk genotype.6
In most patients, the cause of disease is unknown.6 This is classified as sporadic ALS (sALS), and accounts for up to 90% of cases.6 In some 5–10% of patients, ALS is inherited, and is termed familial ALS (fALS).5
Although no environmental risk factors have been proven to be causative of sALS, factors such as smoking, and exposure to agricultural chemicals, heavy metals, low-frequency electromagnetic waves and radiation, have been shown to increase the risk, due to oxidative stress and neuroinflammation.7,9 Evidence also suggests that there may be an increased susceptibility to ALS among athletes when compared with the general population, though physical activity itself is not yet proven to be a cause of disease.9
ALS is thought to be linked to defects in the structure and function of mitochondria, while evidence suggests that inflammatory processes and non-neuronal cells may cause sALS onset.4,7
ALS is believed to be a syndrome that results from the complex interplay between genetic and environmental causes; varied genetic mutations can result in similar phenotypes, while one genetic mutation can result in many different phenotypes.3 Both fALS and sALS can present with common biological and clinical features.8
fALS has an autosomal dominant pattern of inheritance and at least 30 genetic mutations have been found in fALS, sALS or both.1,6,8 Between 30% and 50% of fALS cases are caused by repeat expansions of the C9orf72 gene, which causes accumulation of the dipeptide repeat protein.6 This is also the cause of 7% of sALS cases.6
Mutations of the superoxide dismutase 1 (SOD1) gene have also been found in every exon of patients with ALS ever since their association with the condition was discovered in 1993.10 These mutations account for around 20% of fALS cases, and around 5% of sALS cases.11
Demographics of patients with ALS
Estimated prevalence and incidence
ALS is the most common adult-onset form of motor neuron disease.1,12 The cumulative lifetime risk of ALS is about 1:350 in men and 1:400 in women.6,8 Worldwide, 30,000 patients are estimated to die from ALS every year.5
However, significant geographical differences in prevalence and incidence exist globally: both are higher in developed regions.6,10,13 This may be because population aging is increased in developed regions, and there is a correlation between prevalence and incidence and an aging population.13 It is thought that regional variance observed in disease incidence could also correspond to differences in ancestral origin or environmental exposure. Although, epidemiological homogeneity among European populations has been observed.14
The prevalence of ALS is thought to be around 5–6 per 100,000 people in the United States and Europe.1,12 Worldwide, the prevalence is estimated to be 4.42 per 100,000 and is reported to be higher among males than females (5.96 vs 3.90 per 100,000).13 Across Europe, prevalence of ALS varies from 1.1 per 100,000 in Serbia to 8.2 per 100,000 in the Faroe Islands – similarly, areas in Italy and the Netherlands have high prevalence (8.0/100,000 each).15
The reported worldwide incidence of ALS ranges from 1.7 to 3 per 100,000.6,12 These estimates do vary across Europe, ranging from 0.5 per 100,000 in Serbia to 3.6 per 100,000 in the Faroe Islands: similar rates have been reported for the US and Canada.15 As with prevalence, there is a higher incidence rate among males than females (1.91 vs 1.36 per 100,000).13 In the most at-risk age group (45–75 years), incidence rises to 4–8 per 100,000, which may reflect the aging global population.6,15 European countries with higher latitudes report increased incidence rates: a trend consistent with genetic evidence, suggesting that the risk for ALS is highest in Scandinavian countries and follows a north-to-south gradient.15
Overall, this suggests that 1–2 new patients per 100,000 will be diagnosed with ALS each year in Europe and the United States.8
Age of onset
The mean age of ALS onset is variable, ranging from between 40 and 60 years of age for fALS and 58–63 years for sALS, though geographical variation has been reported.6,16
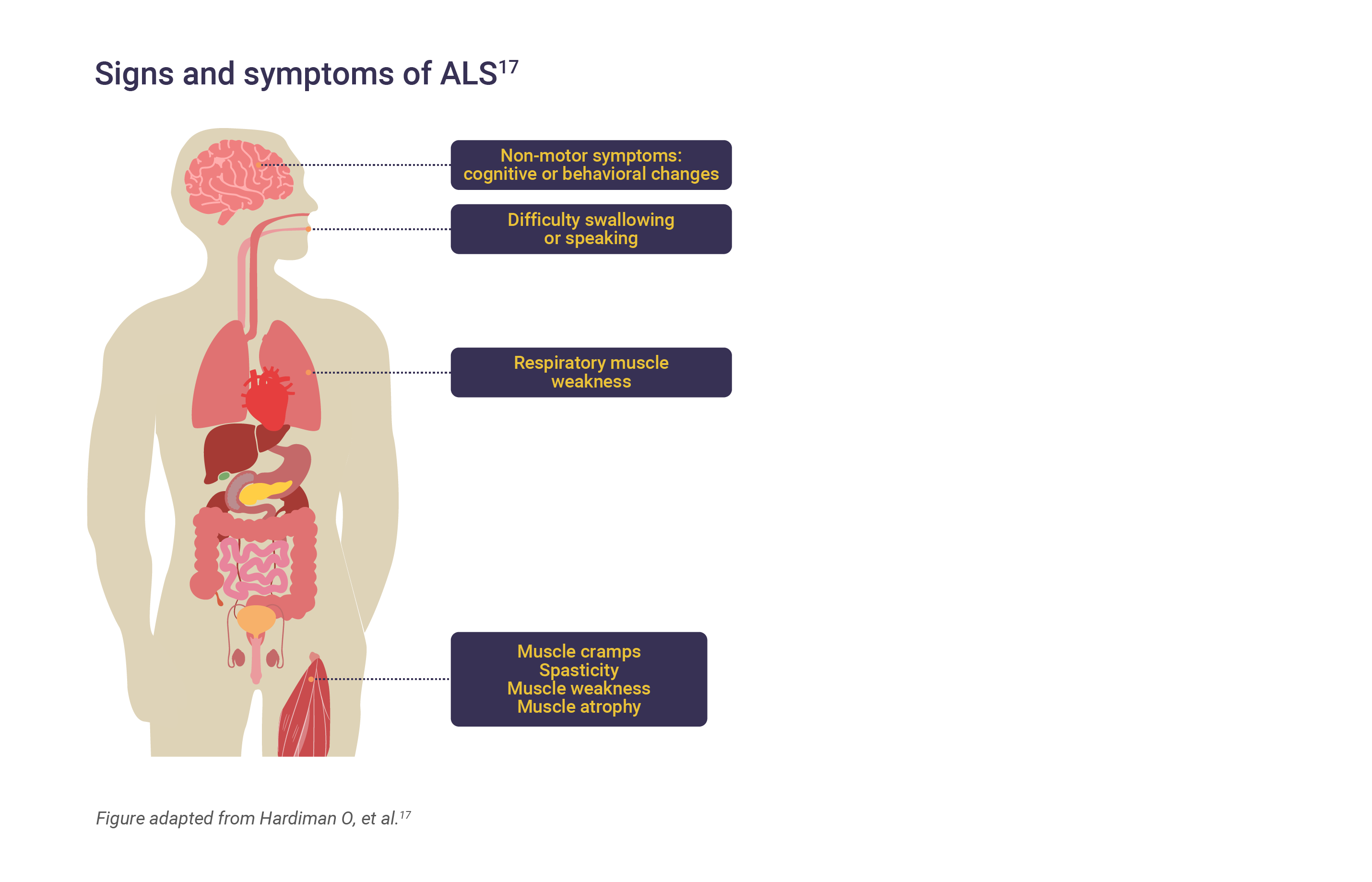
The signs and symptoms of ALS
The clinical presentation of ALS is heterogenous and its phenotype can be categorized as spinal/limb or bulbar-onset, based on clinical characteristics and site of onset.2,4 Approximately two-thirds of patients have spinal-onset disease, which may present with focal limb muscle weakness and wasting.4,6 This most often starts in the limbs, more often in distal muscles than in proximal muscles.6 Spasticity may develop in the weakened atrophic limbs, affecting manual dexterity and gait.4
In an estimated third of cases with bulbar-onset ALS, the first signs of disease may include difficulty chewing, speaking, or swallowing.4,6,8 Patients may also present with limb symptoms.4
Hallmarks of the condition include progressive muscle weakness accompanied by muscle atrophy, fasciculations, muscle cramps, and slowness of movement.6 In later-stage disease, muscles of the eyes and bladder may be affected.8
The onset of weakness in ALS is usually focal and typically spreads to adjacent areas of the body.6 There is usually a recognisable association between spreading of the disease within the motor system and neuroanatomic progression in myotomes and the motor cortex.6 When lower motor neurons become affected they initially show excessive electrical irritability, leading to fasciculations.8 As the neurons degenerate, they lose effector synaptic connectivity and muscle atrophy follows.8
About 50% of patients will suffer from extra-motor manifestations including behavior changes, executive dysfunction and language problems, which are severe enough to be classed as frontotemporal dementia as an additional diagnosis in 10–15% of cases, while mild behavioral or cognitive changes may be reported in 35–40% of cases.6
Determining a diagnosis of ALS
Symptoms of ALS are nonspecific to the disease and may resemble other neuromuscular diseases and diagnostic delays are common, particularly in the early stages of disease onset.16 Early diagnosis can be challenging, especially in cases where motor symptoms are confined to 1 region, or to upper motor neuron (UMN) and lower motor neuron (LMN) systems only.6 A lack of existing confirmatory biomarkers means that clinical progression must usually be observed before confirmation of a diagnosis.16
The time taken to demonstrate this has led to reports of a mean or median diagnostic delay: despite a mean age of onset of ~62 years, the mean age of diagnosis is 64 years and median age is 65 years, demonstrating a mean diagnostic delay of at least 12 months.15
The diagnostic criteria of ALS are:18
- Signs of LMN degeneration by clinical, electrophysiological, or neuropathologic examination
- Signs of UMN degeneration by clinical examination
- Progressive spread of disease in the absence of neuroimaging and electrophysiological evidence of other neuropathologies
A definitive diagnosis is ascertained by:18
- Taking the patient’s history and undertaking appropriate physical and neurological examinations
- Electrophysiological examinations to confirm LMN degeneration in clinically involved regions, identify LMN degeneration in clinically uninvolved regions, and exclude other disorders
- Neuroimaging examinations
- Laboratory tests to rule out disorders that may have similar presentations to ALS
- Neuropathological tests
- Repeated clinical and electrophysiological tests every 6 months to evaluate disease progression
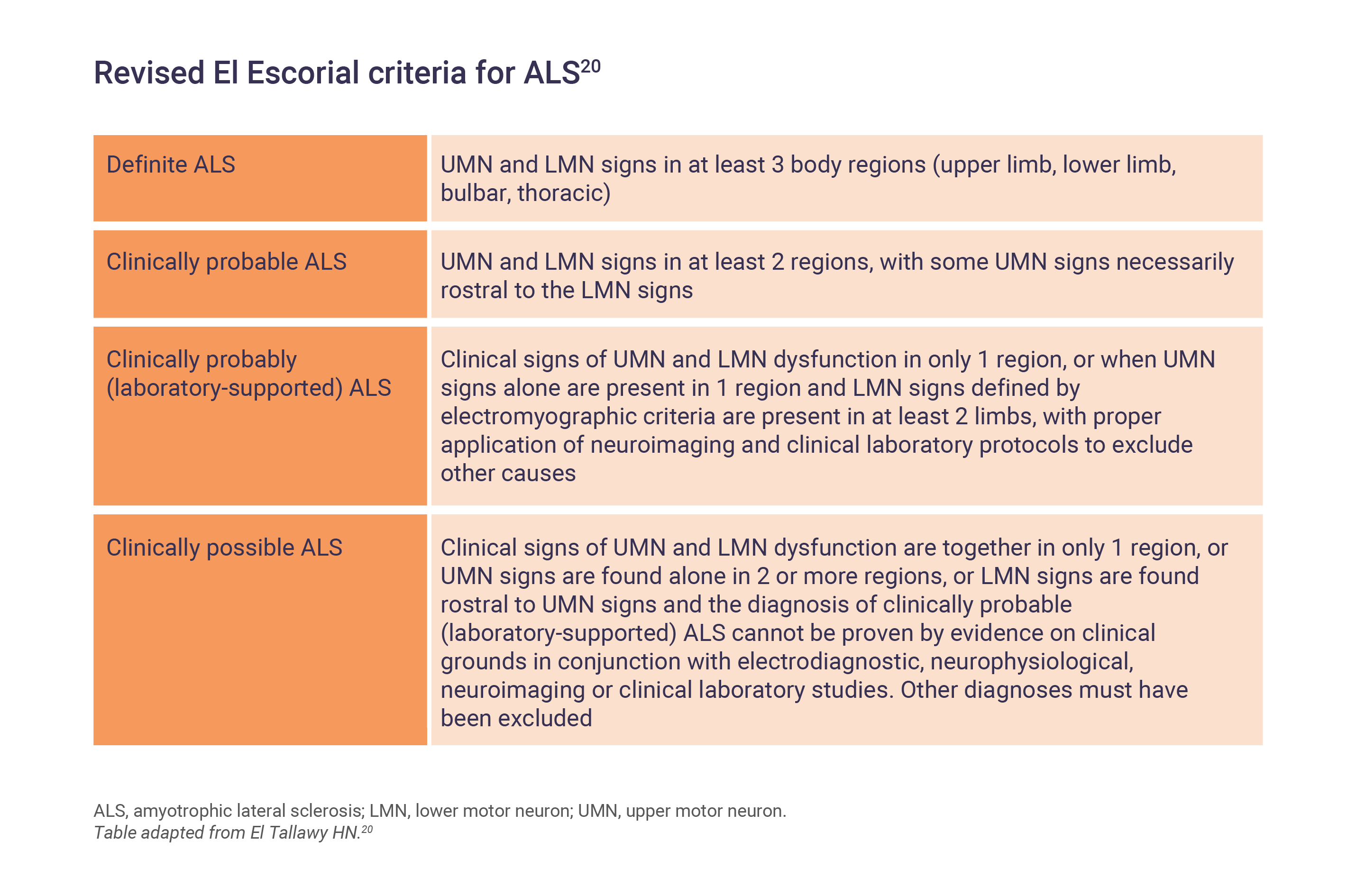
For research purposes, ALS is classified and measured by the revised El Escorial criteria to improve recruitment homogeneity in clinical studies and trials.11,19
As ALS is a rare disorder, other more common diagnoses require consideration and exclusion before a diagnosis can be reached. These include peripheral neuropathy, Lyme disease, vitamin B12 deficiency, thyroid disease, and metal toxicity.8 Reversible motor neuron disorders, such as those typically associated with autoantibodies, should also be excluded before a diagnosis of ALS is made.8
People with bulbar-onset disease can be effectively evaluated using the Awaji criteria, a set of highly sensitive electrodiagnostic tests.11 It should be noted that both the El Escorial and Awaji criteria are limited by a lack of clinical sensitivity and, overall, there is an unmet need for clinical diagnostic criteria of ALS and related subtypes of motor neuron disease, which needs to be addressed to reduce the diagnostic delay.6
What is the prognosis of ALS?
There is variability in the rate of disease progression of ALS;6 however, the course of disease tends to be relentless and usually results in death due to respiratory paralysis 3–5 years from diagnosis.1,8 About 10% of ALS patients are reported to have a slower form of the disease, with survival of 10 years or longer.16
Predictors for longer ALS survival include male sex, younger age at onset and diagnosis, higher body mass index (BMI), slower progression rate, and multidisciplinary clinic attendance.16,21 Improved survival is also associated with spinal (vs bulbar) onset.13,16 Conversely, respiratory or genitourinary comorbidities and the presence of both cognitive impairment and depression have been identified as negative predictors of survival in ALS patients.22,23
The ALS Functional Rating Scale – Revised is a validated questionnaire based scale that can be used clinically to monitor disease progression.24 The questionnaire uses 12 questions of physical function scored between 0 (no function) and 4 (full function) to generate a summary score, defining the functional stage of the patient.24
When respiratory failure can no longer be managed by non-invasive ventilation, patients can undergo tracheotomy to help prolong survival.25 The median survival after tracheotomy is reported to range from 8 months to 3 years.25 However, only 10% of patients in Western countries elect to undergo tracheotomy – this rises to 30% in Japan, suggesting cross-cultural differences in the management of ALS patients.25
ALS, amyotrophic lateral sclerosis; BMI, body mass index; fALS, familial amyotrophic lateral sclerosis; LMN, lower motor neuron; sALS, sporadic amyotrophic lateral sclerosis; SOD1, superoxide dismutase 1; UMN, upper motor neuron.
References
- Wobst HJ, Mack KL, Brown DG, et al. Med Res Rev 2020;40:1352–1384.
- Van Es MA, Hardiman O, Chio A, et al. Lancet 2017;390:2084–2098.
- Grad LI, Rouleau GA, Ravits J, Cashman NR. Cold Spring Harb Perspect Med 2017;7:a024117.
- Wijesekera LC, Leigh PN. Orphanet J Rare Dis 2009;4:3. doi: 10.1186/1750-1172-4-3.
- Petrov D, Mansfield C, Moussy A, Hermine O. Front Aging Neurosci 2017;9:68. doi: 10.3389/fnagi.2017.00068.
- Masrori P, Van Damme P. Eur J Neurol 2020;27:1918–1929.
- Rojas P, Ramírez AI, Fernández-Albarral JA, et al. Front Neurosci 2020;14:566858.
- Brown RH, Al-Chalabi A. NEJM 2017;377:162–172.
- Zarei S, Carr J, Reiley L, et al. Surg Neurol Int 2015;6:171.
- Martin S, Al Khleifat A, Al-Chalabi A. F1000 Research 2017;6:371.
- Kiernan MC, Vucic S, Cheah BC, et al. Lancet 2011;377:942–955.
- Oskarsson B, Gendron TF, Staff NP. Mayo Clin Proc 2018;93:1617–1628.
- Xu L, Liu T, Liu L, et al. J Neurol 2020;267:944–953.
- Leighton DJ, Newton J, Stephenson LJ, et al. J Neurol 2019;266:817–825.
- Chio A, Logroscino G, Traynor BJ, et al. Neuroepidemiology 2013;41:118–130.
- Longinetti E, Fang F. Curr Opin Neurol 2019;32:771–776.
- Hardiman O, Al-Chalabi A, Chio A, et al. Nat Rev Dis Primers 2017;3:17071.
- Criteria for the diagnosis of ALS. ALS Association 2020. Available at https://www.als.org/navigating-als/resources/fyi-criteria-diagnosis-als. [Last accessed: August 2023].
- Brooks BR, Miller RG, Swash M, et al. Amyotroph Lateral Scler & Other Motor Neuron Dis 2000;1:293–299.
- El Tallawy HN (2012) – Motor Neuron Disease. In Zaher A (ed.) Neuromuscular Disorders (pp201–220). London, UK: IntechOpen.
- Dorst J, Chen L, Rosenbohm A, et al. J Neurol 2019;266:1516–1525.
- Bhattacharya R, Harvey RA, Abraham K, et al. Amyotroph Lateral Scler Frontotemporal Degener 2019;20:251–259.
- De Marchi F, Sarnelli MF, Solara V, et al. Acta Neurol Scand 2019;139:438–445.
- Cedarbaum JM, Stambler N, Malta E, et al. J Neurol Sci 1999;169:13–21.
- Spataro R, Bono V, Marchese S, et al. J Neurol Sci 2012;323:66–70.
